Quality of vaccines is important because of the risk of side effects. Pressures for rapid approval of vaccines are potentially conductive to the distribution of preparations having suboptimal and/or unstable quality. Preparations submitted for official approval are not necessarily the same quality as those administered to the public, which depends on the manufacturing standards. There have been reports on cardiovascular, clotting-related and other adverse events after vaccinations with Gam-COVID-Vac vaccine [1–12]. Reportedly, most adverse effects were mild [8]. In a retrospective study encompassing 6600 participants, Gam-COVID-Vac had the highest rate of adverse effects, the overall percentage being 82.7 % [12]. Regarding the studies reporting Gam-COVID-Vac effectiveness, ambiguities have been noticed and objections raised [9,13]. Of particular importance has been myocarditis, which can cause dilated cardiomyopathy [14,15]. The number of unreported adverse effects is unknown. The topic seems to be blanketed; serious adverse effects are sweepingly denied by some handbooks [16].
We have found in the literature no information on herpes zoster (HZ) after the vaccination with Gam-COVID-Vac. HZ has been reported following COVID-19 vaccination with other vaccines, although conclusive evidence of a direct link is lacking. A serious post-vaccination event is Varicella-Zoster meningitis [17,18]. Reports on side effects of renowned vaccines do not imply higher risks but indicate that they are thoroughly studied. Complications after the use of foreign vaccines have been reviewed without mentioning those from domestic products [19]. Some official information is neither transparent nor trusted [20].
Health workers’ rights are sometimes violated during pandemics [21], in particular, the principle of informed consent. Compulsory Covid-19 vaccinations of medical personnel, teachers and some other employees have been criticized. Under threat of suspension from work some of them would conceal contraindications [22]. The compulsory vaccination involved those who already had COVID-19 infection. Admittedly, other events are in the foreground today; and Covid-19 vaccinations are not required anymore. Although SARS-CoV-2 vaccinations are generally regarded to be safe, concerns are backed by increasing numbers of reports on moderate-to-severe side effects [23]. Children, young adults and many other people can mount their own immunity to SARS-CoV2 undergoing acceptably low risk. There is an opinion that it is unethical to impede the access to natural immunity [24]. In particular, the “vaccination of COVID-recovered individuals should be subject to clinical equipoise and individual preference” [25]. In future, the increase in mortality due to different factors might be ascribed to COVID -19, and subsequent mortality decrease — to “successful” anti-epidemic measures including vaccinations. Adverse effects of vaccinations may be misattributed to the COVID-19 infection or other causes [26].
Case report
A 65-years-old male was vaccinated with Gam-COVID-Vac one year after a nearly asymptomatic COVID-19 infection, and thereafter noticed cardiac arrhythmia, dyspnea and erythematous, in places vesicular eruption on the chest, compatible with HZ. Intense headache was the leading symptom immediately after both vaccine doses. A further development has been observed at the patient’s age 69: relapse of HZ with typical eruption on the right arm and shoulder (Fig. 1). The most disturbing symptom was pain in the area of shoulder joint irradiating down to the thumb. Later on, the pain maximum relocated to the wrist joint. The patient was examined at the Dermatovenereological dispensary in Moscow and the treatment prescribed: Valaciclovir 1,000 mg orally three times a day for 7 days; topically Fucorcin (antiseptic solution containing boric acid, phenol, resorcinol and basic fuchsine). The treatment has been followed by a visible improvement of skin lesions, although the pain is persisting i. e. postherpetic neuralgia developed. One lesion in the cubital fossa suppurated. The doctor in charge was informed about development of symptoms after the Gam-COVID-Vac vaccination but this information has not been recorded.
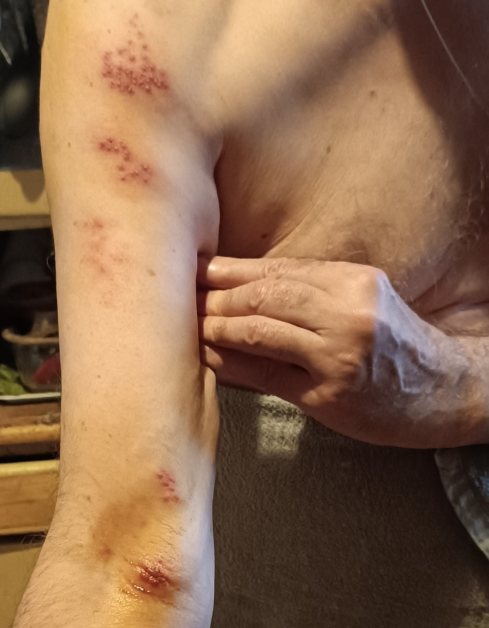
Fig. 1. HZ lesions ~5 days old
Discussion and conclusion
HZ following COVID-19 vaccination with different vaccines has been reported although conclusive evidence of a direct link is lacking. According to retrospective analyses and systematic reviews, the vaccination has been associated with increased risk of HZ [17,27–29]. In subgroup analysis, the mRNA vaccination was associated with a higher risk of HZ compared with the adenovirus vector vaccines [29]. As for mechanisms, changes in the host’s immune status can result in a failure to suppress herpesvirus replication [17,28]. Admittedly, some studies have failed to identify an association between COVID-19 vaccination and HZ. The number of unreported cases is unknown. The documentation reliability of side effects remains questionable. The rarity of reports on the side effects of COVID-19 vaccinations may be caused by policies discouraging such reporting [30]. The information about association of symptoms with vaccination is sometimes left unrecorded.
In conclusion, premature approval of vaccines due to ambitions and rivalries without long-term safety data should be avoided. Further research, especially that on long-term risks of various vaccine types, is needed. The natural process of ageing is associated with a reduction in cellular immunity, which may predispose to HZ; references are in [31]. Aged people sometimes pay for victories in the vaccine race and other rivalries, which is roughly reflected by life expectancy statistics.
References:
- Bludau H-B, Jargin SV. COVID-19 and Vaccination. In: Selected Aspects of Healthcare in Russia. Paperback and eBook. Cambridge Scholars Publishing; 2025, pp. 98–110.
- Bludau HB, Jargin SV. Post-COVID-19 syndrome vs. consequences of vaccination with special reference to cardiovascular conditions. Scibase Cardiol. 2023;1:1002.
- Chahed F, Ben Fadhel N, Maamri K, et al. An unusual occurrence of autoimmune pancreatitis after gam-Covid-Vac (Sputnik V): A case report and literature review. Br J Clin Pharmacol. 2023;89:2915–9.
- Денисенко А. С., Рисс М. Е., Кропачев И. Г., Никитина Н. Н. Первые случаи коагуляционных нарушений как осложнения после введения вакцины gam-covid-vac (Sputnik V) vaccine. Вестник НовГУ. 2021;(3):61–64.
- Ptushkin V, Arshanskaya E, Vinogradova O, et al. The features of COVID-19’s course and the efficacy of the GamCOVID-Vac vaccine in patients with paroxysmal nocturnal hemoglobinuria. Hematol Rep. 2023;15:503–12.
- Рачин А. П., Котова О. В., Демьяновская Е. Г., Крыжановский С. М., Пикус Л. Е. COVID-19 и постковидный синдром: руководство для невролога. М.: АБВ-пресс; 2023.
- Lipp HP. Impfstoffe gegen „coronavirus disease 2019“ (COVID-19): Wirksamkeitsvergleich, Sicherheitsaspekte und aktuelle Herausforderungen. Inn Med (Heidelb). 2022;63:666–79.
- Baraniuk C. Covid-19: What do we know about Sputnik V and other Russian vaccines? BMJ. 2021;372:n743.
- Hasanzarrini M, Salehi AM, Nirumandi Jahromi S. Development of peptic ulcer following second shot of sputnik v vaccine: a case report and literature review of rare side effects of Sputnik V vaccine. Case Rep Infect Dis. 2023;2023:9989515.
- Mirmosayyeb O, Barzegar M, Rezaei M, et al. Bell's palsy after Sputnik V COVID-19 (Gam-COVID-Vac) vaccination. Clin Case Rep. 2022;10(2):e05468.
- Ameri M, Abolmaali M, Alwedaie SMJ, et al. Severe persistent eczema in a recipient of the Gam-COVID-Vac vaccine. Eur J Case Rep Intern Med. 2022;9(1):003042.
- Babaee E, Amirkafi A, Tehrani-Banihashemi A, et al. Adverse effects following COVID-19 vaccination in Iran. BMC Infect Dis. 2022;22(1):476.
- Bucci EM, Berkhof J, Gillibert A, et al. Data discrepancies and substandard reporting of interim data of Sputnik V phase 3 trial. Lancet. 2021;397:1881–3.
- Благова О. В., Коган Е. А. Миокардит в период пандемии SARS-CoV-2. М.: Практическая медицина; 2023.
- Naghashzadeh F, Shafaghi S, Dorudinia A, et al. Myocarditis following rAd26 and rAd5 vector-based COVID-19 vaccine: case report. ESC Heart Fail. 2022;9(2):1483–6.
- Костинов М. П. Вакцинопрофилактика COVID-19 у пациентов с коморбидными заболеваниями. М. Группа МДВ; 2022.
- Martora F, Megna M, Battista T, et al. Viral reactivation following COVID-19 vaccination: a review of the current literature. Clin Exp Dermatol. 2024;49:556–65.
- Lazzaro DR, Ramachandran R, Cohen E, Galetta SL. Covid-19 vaccination and possible link to Herpes zoster. Am J Ophthalmol Case Rep. 2022;25:101359.
- Халимова Х. М., Рашидова Н. С., Салимжонов Ж. Ж. Неврологические осложнения после вакцинации COVID-19. Журнал неврологии и психиатрии им. С. С. Корсакова. 2023;123(12):13–19.
- King EJ, Dudina VI. COVID-19 in the Russian Federation. In: Greer SL, King EJ, da Fonseca EM, eds. Coronavirus politics: the comparative politics and policy of COVID-19. University of Michigan Press; 2021, pp. 436–451.
- Sheather J, Hartwell A, Norcliffe-Brown D. Serious violations of health workers’ rights during pandemic. BMJ. 2020;370:m2824.
- Понкин И. В. О массовой неизбирательной принудительной вакцинации. Вопросы культурологии. 2022;(2):120–40; продолжение (3):222–35.
- Finsterer J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol Scand. 2022;145:5–9.
- Hart EM. Is it ethical to impede access to natural immunity? The case of SARSCoV2. Rapid Response Re. Mahase E. Covid-19: UK starts social distancing after new model points to 260 000 potential deaths. BMJ 2020;368:m1089. https://www.bmj.com/content/368/bmj.m1089/rr-6
- Shenai MB, Rahme R, Noorchashm H. Equivalency of protection from natural immunity in COVID-19 recovered versus fully vaccinated persons: A systematic review and pooled analysis. Cureus. 2021;13:e19102.
- Mead MN, Seneff S, Wolfinger R, et al. COVID-19 mRNA vaccines: lessons learned from the registrational trials and global vaccination campaign. Cureus. 2024;16(1):e52876.
- Martinez-Reviejo R, Tejada S, Adebanjo GAR, et al. Varicella-Zoster virus reactivation following severe acute respiratory syndrome coronavirus 2 vaccination or infection: New insights. Eur J Intern Med. 2022;104:73–79.
- Maple PAC. COVID-19, SARS-CoV-2 vaccination, and human herpesviruses infections. Vaccines (Basel). 2023;11(2):232.
- Chen IL, Chiu HY. Association of herpes zoster with COVID-19 vaccination: A systematic review and meta-analysis. J Am Acad Dermatol. 2023;89(2):370–1.
- Joob B, Wiwanitkit V. Acute myocarditis after coronavirus disease 2019 vaccination. Anatol J Cardiol. 2021;25:841–2.
- de Oliveira Gomes J, Gagliardi AM, Andriolo BN, et al. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst Rev. 2023;10(10):CD008858.

