The study investigates an electrocoagulation process utilizing an iron electrode to effectively remove copper from electroplating wastewater. Experiments evaluated the influence of pH, current density, electrode spacing, and electrolyte dosage on removal efficiency. Optimal conditions were identified at a pH of 5, an inter-electrode distance of 1 cm, a current density of 100 mA/cm², and a sodium sulfate (Na₂SO₄) electrolyte dosage of 3 g/L. Under these conditions, a copper removal efficiency of 98.4 % was achieved with a low energy consumption of 0.0094 kWh. These findings highlight the process's potential as an energy-efficient and environmentally sustainable solution for treating heavy metal-contaminated wastewater.
Keywords: electrocoagulation, electroplating wastewater, copper, energy consumption
- Introduction
Electroplating operations significantly contribute to environmental and waterway pollution by generating hazardous wastewater laden with toxic heavy metals, posing severe risks to ecosystems and aquatic life. This wastewater is highly acidic, exhibits strong ionic content, and often contains valuable metals such as gold, silver, copper, nickel, and zinc, necessitating advanced treatment to mitigate environmental harm. Several conventional processes including neutralization followed by precipitation, or physical treatment including ion-exchange, adsorption, filtration, and chemical coagulation and membrane technology are also used for wastewater treatment. Nevertheless, this process results in producing large amounts of precipitated metallic sludge that requires additional handling and cost. Therefore, electrocoagulation process is an alternative promising solution using electricity instead of expensive chemical reagents. The advantages of this processes compared to the other conventional methods is that it uses simple equipment, is easy to operate, shorter time is allowed, and limited chemicals are needed. Moreover, this process provides rapid sedimentation of the electro-generated flocs besides producing less sludge.
The aim of this research was to investigate the possibility of copper removal in electroplating wastewater under different operational variables (pH, current density, inter-electrode distance and the dosage of electrolyte) from the aqueous solution by applying the EC method.
- Materials and methods
2.1 Chemicals
Electroplating wastewater was collected from Quang Vinh metal plating Co.LTD. NaOH, H 2 SO 4 , and Na 2 SO 4 (Xichlong, China) were used to prepare aqueous solutions for the experiments. The pH of the solutions was adjusted by adding 0,1 M HCl or 0,1 M NaOH.
2.2 Experimental procedures
Laboratory-scale electrocoagulation (EC) experiments were conducted in a 1L glass cell (10 mm width × 12 mm length × 15 mm height) equipped with two parallel monopolar iron electrodes (2 mm × 7 mm × 17 mm) serving as anode and cathode. A digital AC power supply (Itech IT6952A, 0–60 V, 0–10 A, 600 W) provided alternating current to the EC cell. Prior to each experiment, the iron electrodes were cleaned with 0.1 M HCl to remove surface impurities, rinsed with distilled water, dried, and weighed. Experiments were performed at ambient temperature (25–27°C) using 1L of aqueous solution over 20 minutes. Post-treatment, the solution was filtered, the filtrate analyzed for pollutant removal, and the electrodes were dried and reweighed to assess material loss. This setup ensures precise control and reproducibility, making it suitable for evaluating EC’s efficacy in treating wastewater contaminants.
2.3 Analytical method
The copper in electroplating wastewater was analyzed using flame atomic absorption spectrometry (AAS) SavantAA Σ, GBC, Australia. pH of solution was conducted by Milwaukee, Poland.
The efficiency of copper removal and electrode consumption were calculated using the following equations:
Cu
2+
removal (%) =
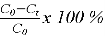
Where: C 0 : the initial Cu 2+ concentration (mg/L) and C t : the mole of Cu 2+ after treatment (mg/L)
Electronical consumption (kWh)W =
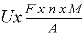
Where: A– is the molecular weight of iron; n- is the number of electron involved, and F is the faraday constant (96485.3 C mol -1 ); m — the weight of iron dissovle (g)
-
Results and discussion
- Effect of pH
To assess its impact, experiments were conducted with a 1 cm electrode gap, using 200 mg/L electroplating wastewater with copper, and initial pH values ranging from 3 to 9. Results from Fig. 1 indicate that copper removal efficiency increased with rising pH, peaking at 90.1 % at pH 7. This enhanced efficiency at higher pH levels is attributed to increased hydrogen generation at the cathode and the release of hydroxide ions from copper hydroxide at pH ≥ 7. However, a pH of 5 was determined to be optimal for this electrocoagulation process, balancing efficiency and operational stability.
When iron is used as anode, the reactions are as follows:
At the cathode,
2H 2 O+2e − →H 2 (g)+2OH − (1)
At the anode,
Fe → Fe²⁺ + 2e⁻ (2)
In the solution,
4Fe²⁺ + O₂ + 2H₂O → 4Fe³⁺ + 4OH⁻ (3) [2]
These Fe²⁺ and Fe³⁺ ions react with OH⁻ to form iron hydroxides (Fe(OH)₂ and Fe(OH)₃), which act as coagulants, adsorbing and precipitating heavy metal ions like Cu²⁺ as Cu(OH)₂ or complexes (Cu²⁺ + Fe(OH)₃ → Cu(OH)₂↓ + Fe³⁺), or reducing Cu²⁺ to metallic copper at the cathode (Cu²⁺ + 2e⁻ → Cu↓)
3.2 Effect of current density
The study evaluated current densities at 20, 40, 60, 80, and 100 mA/cm², with results depicted in Figure 2, under conditions of pH 5, a 1 cm inter-electrode distance, and 20 minutes of electrolysis. Figure 2 illustrates that copper removal efficiency rises from 72.1 % to 93.4 % as current density increases from 20 to 100 mA/cm². This enhancement occurs because higher current densities promotes greater anodic dissolution of iron, producing more hydroxide flocs that effectively capture pollutants. Additionally, increased current density accelerates bubble generation while reducing bubble size, enhancing pollutant removal through improved H 2 flotation.
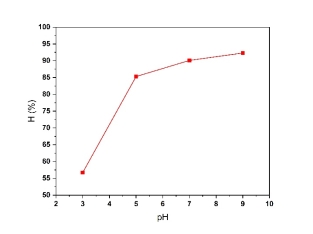
Fig. 1. The effect of pH
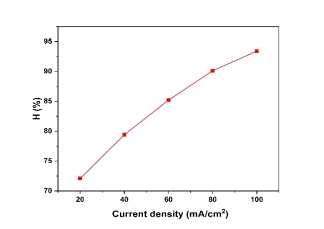
Fig. 2. The effect of current density
- Effect of inner- electrode distance
The fig. 3 was illustrated the effect of distance between two electrodes by the experimental conditions: pH 5, current density: 100mA/cm 2 in 20 min. The results showed the further the distance, the fewer efficiency of copper removal in electroplating wastewater. As the distance between electrodes becomes lower, more electrochemically generated gas bubbles bring about turbulent hydrodynamics, thereby leading to a high mass transfer as well as to a high reaction rate between the coagulant species and pollutants. In addition, inter-electrode gap defines the residence time between the anode and the cathode for a continuous system and the time of treatment for a batch reactor for reaching a desirable EC efficiency [3]
- The effect of electrolyte (Na 2 SO 4 )
The removal efficiency for copper was 94.2 to 98.6 % for 1 to 3 of Na 2 SO 4 respectively. Because Na 2 SO 4 is a strong electrolyte. In electrocoagulation using iron electrodes, Na₂SO₄ is employed as an electrolyte due to its ability to enhance solution conductivity, minimize undesirable side reactions, and support efficient coagulant formation. When dissolved, Na₂SO₄ dissociates into Na⁺ and SO₄²⁻ ions, reducing solution resistance and lowering energy consumption during electrolysis. At the iron anode, oxidation produces Fe²⁺ or Fe³⁺ ions, which react with OH⁻ ions generated at the cathode to form iron hydroxides (e.g., Fe(OH)₂ or Fe(OH)₃), effective coagulants for pollutant removal. Unlike NaCl, Na₂SO₄ avoids producing harmful chlorine gas or chlorinated byproducts, reducing equipment corrosion and environmental risks. Additionally, Na₂SO₄ is cost-effective, environmentally benign, and maintains stable pH conditions, optimizing the coagulation process.
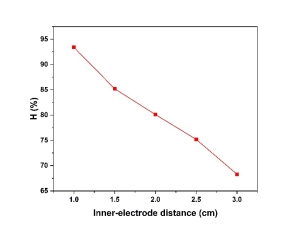
Fig. 3. Effect of inner-electrode distance
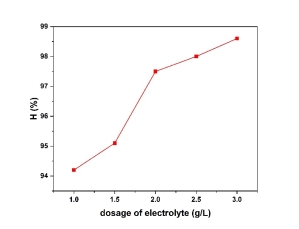
Fig. 4. Effect of the dosage of electrolyte
- The consumption of electricity
When the pH was at 5, the inner electrode distance was 1 cm, the current density was 100 mA/cm 2 , and the mNa 2 SO 4 concentration was 3g/L, the copper removal efficiency in electroplating wastewater was 98.4 %. The total iron weight for dissolving was 0.3 g, and the electricity usage was 0.0094 kWh.
Conclusion
The findings indicate that when iron alloy was used as the anode and cathode, the maximum removal efficiency of 98.4 % was attained at a current density of 100 mA/cm 2 , pH of 5, inter-electrode distance of 1 cm, and dosage of Na 2 SO 4 3g/L. Compared to previous studies, the electricity consumption was 9.4 Wh more economical.
References:
- Y. Yavuz, Ü. B. Ögütveren, Treatment of industrial estate wastewater by the application of electrocoagulation process using iron electrodes, Journal of Environmental Management, 207,2018,
- N. Huda, A.A. A. Raman, M. M. Bello, S. Ramesh, Electrocoagulation treatment of raw landfill leachate using iron-based electrodes: Effects of process parameters and optimization, Journal of Environmental Management, 204(1), 2017
- Aatif Ali Shah, Sunil Walia, Hossein Kazemian, Advancements in combined electrocoagulation processes for sustainable wastewater treatment: A comprehensive review of mechanisms, performance, and emerging applications, Water Research, 252,2024,121248
- Tabash, I., Elnakar, H. & Khan, M. F. Optimization of iron electrocoagulation parameters for enhanced turbidity and chemical oxygen demand removal from laundry greywater. Sci Rep, 14, 16468, 2024.

